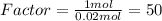Chemistry, 01.10.2019 18:20 poptropic9207
Epsom salts is a hydrated ionic compound with the following formula: mgso4⋅xh2o a sample of epsom salts with a mass of 4.93 g is heated to drive off the water of hydration. the mass of the sample after complete dehydration is 2.41 g. find the number of waters of hydration (x) in epsom salts

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Smog is the term used to describe the combination of fog and smoke
Answers: 1

Chemistry, 22.06.2019 02:30
24 points and brainliest to anyone who can answer under 10 minutes with best ! the table below shows the role of different substances during photosynthesis. substance role during photosynthesis glucose stores chemical energy water combines with glucose to form carbon dioxide chlorophyll traps sunlight which of the following statements would correct one of the roles listed in the table? glucose combines with carbon to form water. chlorophyll reacts with light to produce carbon dioxide. water combines with carbon dioxide during photosynthesis. chlorophyll stores chemical energy needed for photosynthesis.
Answers: 1

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1
You know the right answer?
Epsom salts is a hydrated ionic compound with the following formula: mgso4⋅xh2o a sample of epsom s...
Questions




















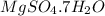
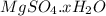 .After complete dehydration we have 2.41 g of
.After complete dehydration we have 2.41 g of 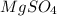 .
.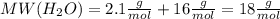
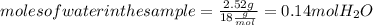
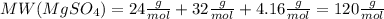
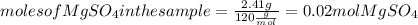
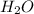 . Let´s find the multiplying factor to obtain the forula for 1 mol of
. Let´s find the multiplying factor to obtain the forula for 1 mol of