
Chemistry, 01.10.2019 19:00 kayyjayy3106
The work function of an element is the energy required to remove an electron from the surface of the solid element. the work function for lithium is 279.7 kj/mol (that is, it takes 279.7 kj of energy to remove one mole of electrons from one mole of li atoms on the surface of li metal). what is the maximum wavelength of light that can remove an electron from an atom on the surface of lithium metal?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
The table lists pressure and volume values for a particular gas. which is the best estimate for the value of v at p = 7.0 × 103 pascals?
Answers: 3

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2

Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3
You know the right answer?
The work function of an element is the energy required to remove an electron from the surface of the...
Questions

Arts, 30.06.2019 17:30

Chemistry, 30.06.2019 17:30

History, 30.06.2019 17:30

Mathematics, 30.06.2019 17:30

Mathematics, 30.06.2019 17:30

Mathematics, 30.06.2019 17:30




History, 30.06.2019 17:30



Mathematics, 30.06.2019 17:30

Chemistry, 30.06.2019 17:30

History, 30.06.2019 17:30


Mathematics, 30.06.2019 17:30

Mathematics, 30.06.2019 17:30

Biology, 30.06.2019 17:30

History, 30.06.2019 17:30

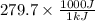
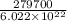
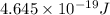
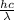
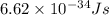
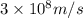
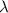 = wavelength
= wavelength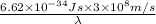
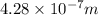
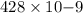 m
m

