
Chemistry, 01.10.2019 20:30 anikabarnhart
Consider the following reaction representing the combustion of propane: + o2 → co2 + h2o a. balance the equation b. how many moles of oxygen are required to burn 1 mol of propane? c. how many grams of oxygen are required to burn 100 g of propane? d. at standard temperature and pressure, what volume of oxygen would be required to burn 100 g of propane? if air is 21 percent oxygen, what volume of air at stp would be required? e. at stp, what volume of carbon dioxide would be produced when 100 g of propane are burned? f. the standard enthalpy of propane is -103.8 kj/mol. find the gross heat released when 1 kg is burned.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Consider the point on the plot where 10.0 g of naoh have been added. what amount of naoh, in moles, has been added? 0.308 mol fecl3 initially present
Answers: 1

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1

Chemistry, 22.06.2019 12:00
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1
You know the right answer?
Consider the following reaction representing the combustion of propane: + o2 → co2 + h2o a. balanc...
Questions






Computers and Technology, 09.03.2020 23:43






Computers and Technology, 09.03.2020 23:44








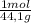 ×
×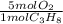 ×
×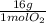 = 181 g of O₂
= 181 g of O₂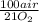 = 1210 L of air
= 1210 L of air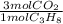 = 6,80 moles of CO₂
= 6,80 moles of CO₂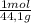 = 22,68 moles
= 22,68 moles


