
Chemistry, 01.10.2019 21:10 ofcitsnijah
Kcl and kbr are both ionic solids. a mixture of kcl and kbr has a mass of 3.595 g. when this mixture is heated in the presence of excess cl2, all of the kbr is converted to kcl. if the total mass of kcl present after this reaction is 3.129 g, what percentage (by mass) of the original mixture was kbr? (hint: be sure that you understand why the mass of the sample has decreased. it may if you write an equation for the reaction that converted the kbr to kcl.)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1

Chemistry, 22.06.2019 08:40
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1

Chemistry, 22.06.2019 14:00
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1

Chemistry, 22.06.2019 17:00
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3
You know the right answer?
Kcl and kbr are both ionic solids. a mixture of kcl and kbr has a mass of 3.595 g. when this mixture...
Questions

Mathematics, 10.05.2021 20:50




Chemistry, 10.05.2021 20:50


Social Studies, 10.05.2021 20:50

History, 10.05.2021 20:50


Mathematics, 10.05.2021 20:50


Mathematics, 10.05.2021 20:50

Geography, 10.05.2021 20:50

Mathematics, 10.05.2021 20:50

Chemistry, 10.05.2021 20:50

Mathematics, 10.05.2021 20:50

Mathematics, 10.05.2021 20:50

Advanced Placement (AP), 10.05.2021 20:50

Mathematics, 10.05.2021 20:50

Mathematics, 10.05.2021 20:50

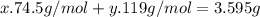
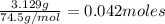
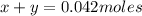
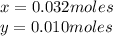
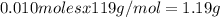
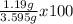 %
% 

