
Chemistry, 01.10.2019 22:00 manarsadi6
The decomposition of nobr is studied manometrically because the number of moles of gas changes; it cannot be studied colorimetrically because both nobr and br2 are reddish-brown. 2nobr(g) →2no(g) + br2(g) use the data below to make the following determinations: (a) the average rate of decomposition of nobr over the entire experiment. (b) the average rate of decomposition of nobr between 2.00 and 4.00 seconds. time (s) [nobr] (mol/l)0.00 0.01002.00 0.00714.00 0.00556.00 0.00458.00 0.003810.00 0.0033the rates of decomposition of nobr are

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Agas is contained in a thick walled balloon when the pressure changes from 1.21 atm to 2.52 the volume changes from 3.75 l to 1.72 l and the temperature change from 293k to blank k
Answers: 3

Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 10:30
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2
You know the right answer?
The decomposition of nobr is studied manometrically because the number of moles of gas changes; it...
Questions

Chemistry, 27.06.2019 12:30


Biology, 27.06.2019 12:30

History, 27.06.2019 12:30


Advanced Placement (AP), 27.06.2019 12:30

Biology, 27.06.2019 12:30


History, 27.06.2019 12:30


Chemistry, 27.06.2019 12:30

Mathematics, 27.06.2019 12:30

History, 27.06.2019 12:30

History, 27.06.2019 12:30


English, 27.06.2019 12:30


Physics, 27.06.2019 12:30


History, 27.06.2019 12:30

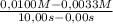 = 6,7x10⁻⁴M/s
= 6,7x10⁻⁴M/s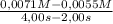 = 8,0x10⁻⁴M/s
= 8,0x10⁻⁴M/s

