
Chemistry, 01.10.2019 22:20 savannahckatz
Apure gold ring (c = 0.128 j/g°c) and pure silver ring (c = 0.235 j/g°c) have a total mass of 16.891 g . the two rings are heated to 66.887 oc and dropped into a 13.9 ml of water at 21.9 oc. when equilibrium is reached, the temperature of the water is 24.2 oc. what is the mass of gold ring? (assume a density of 0.998 g/ml for water.)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:00
19. at high pressures, how does the volume of a real gas compare with the volume of an ideal gas under the same conditions, and why? eman- it is much less because real gas partides are not moving. there is no difference because the gas laws are always obeyed. it is much less because at high pressures the temperature drops. it is much greater because real gas partides take up space.
Answers: 1

Chemistry, 22.06.2019 03:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2


Chemistry, 22.06.2019 21:00
Which answer tells the reason the earth’s climate is getting warmer? too many animals are becoming extinct. large glaciers are melting in antarctica. the earth is moving closer to the sun. driving cars gives off gases that trap heat in the atmosphere.
Answers: 1
You know the right answer?
Apure gold ring (c = 0.128 j/g°c) and pure silver ring (c = 0.235 j/g°c) have a total mass of 16.891...
Questions

History, 14.01.2021 06:20

Arts, 14.01.2021 06:20



Mathematics, 14.01.2021 06:20

Mathematics, 14.01.2021 06:20


History, 14.01.2021 06:20

History, 14.01.2021 06:20

Spanish, 14.01.2021 06:20

History, 14.01.2021 06:20

Mathematics, 14.01.2021 06:20

Mathematics, 14.01.2021 06:20


English, 14.01.2021 06:20

Mathematics, 14.01.2021 06:20




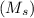 + mass of gold ring
+ mass of gold ring 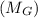
![[M_{G} \times 0.128 J/g^{o}C \times (66.887 - 24.2)^{o}C] + [M_{s} \times 0.235 J/g^{o}C \times (66.887 - 24.2)^{o}C]](/tpl/images/0281/0300/1110a.png) =
= 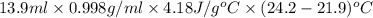
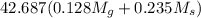 = 133.368
= 133.368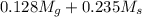 = 3.124
= 3.124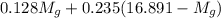 = 3.124
= 3.124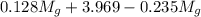 = 3.124
= 3.124 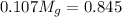
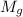 = 7.897 g
= 7.897 g

