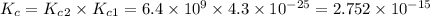Chemistry, 01.10.2019 23:00 morgaaaan651
The following reactions have the indicated equilibrium constants at a particular temperature: n2(g) + o2(g) ⇌ 2no(g)kc = 4.3 × 10−25 2no(g) + o2(g) ⇌ 2no2(g)kc = 6.4 × 109 determine the value of the equilibrium constant for the following equation at the same temperature: n2(g) + 2o2(g) ⇌ 2no2(g) kc = × 10 (enter your answer in scientific notation.)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1

Chemistry, 23.06.2019 00:00
Total the mass on the syringe. record it in the correct row of the data table. kg done click and drag weights to change the pressure. click the syringe to zoom in and see the volume. intro
Answers: 3

Chemistry, 23.06.2019 00:10
Find the missing probability in the table below a.0.10 b.40 c.0.80 d. 0.20
Answers: 2

Chemistry, 23.06.2019 01:10
Volume is a measurement of how fast particles of a substance are moving
Answers: 3
You know the right answer?
The following reactions have the indicated equilibrium constants at a particular temperature: n2(g)...
Questions

Biology, 03.10.2019 13:10



Mathematics, 03.10.2019 13:10



Spanish, 03.10.2019 13:10

Social Studies, 03.10.2019 13:10


History, 03.10.2019 13:10



Physics, 03.10.2019 13:10

English, 03.10.2019 13:10




Mathematics, 03.10.2019 13:10

Mathematics, 03.10.2019 13:10

English, 03.10.2019 13:10

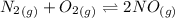
![K_c_1=\frac {[NO]^2}{[N_2][O_2]}=4.3\times 10^{-25}](/tpl/images/0281/1482/cff32.png)
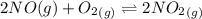
![K_c_2=\frac {[NO_2]^2}{[NO]^2[O_2]}=6.4\times 10^{9}](/tpl/images/0281/1482/9df1f.png)
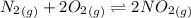
![K_c=\frac {[NO_2]^2}{[N_2][O_2]^2}](/tpl/images/0281/1482/1467b.png)
![[NO]^2](/tpl/images/0281/1482/b4f90.png) and rearranging in the above equation as:
and rearranging in the above equation as:![K_c=\frac {[NO_2]^2}{[NO]^2[O_2]}\times \frac {[NO]^2}{[N_2][O_2]}](/tpl/images/0281/1482/822cf.png)