
Chemistry, 02.10.2019 00:30 XxKaitlynnxX
At equilibrium, the concentrations in this system were found to be [n2]=[o2]=0.100 m and [no]=0.400 m. n2(g)+o2(g)↽−−⇀2no(g) if more no is added, bringing its concentration to 0.700 m, what will the final concentration of no be after equilibrium is re‑established?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1

Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1

Chemistry, 22.06.2019 23:30
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1
You know the right answer?
At equilibrium, the concentrations in this system were found to be [n2]=[o2]=0.100 m and [no]=0.400...
Questions





Mathematics, 25.02.2020 04:48


Mathematics, 25.02.2020 04:48


Mathematics, 25.02.2020 04:48

Mathematics, 25.02.2020 04:48



Mathematics, 25.02.2020 04:48


Mathematics, 25.02.2020 04:49

Biology, 25.02.2020 04:49


English, 25.02.2020 04:49


Advanced Placement (AP), 25.02.2020 04:49

![K = \frac{[C]^{c}.[D]^{d}}{[A]^{a}.[B]^{b}}](/tpl/images/0281/3856/d6241.png)
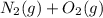 ⇄ 2 NO (g), the equilibrium constant could be calculated as well:
⇄ 2 NO (g), the equilibrium constant could be calculated as well:![K = \frac{[NO]^{2}}{[O_{2}].[N_{2}]}](/tpl/images/0281/3856/92022.png)
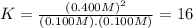
![[N_{2}] = 0.100 M; [O_{2}] = 0.100 M; [NO] = 0.700 M](/tpl/images/0281/3856/59e72.png)
![[N_{2}] = 0.100 M + x; [O_{2}] = 0.100 M + x; [NO] = 0.700 M - 2x](/tpl/images/0281/3856/782eb.png)
![K = \frac{[NO]^{2}}{[O_{2}].[N_{2}]} = 16](/tpl/images/0281/3856/cb079.png)
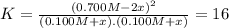
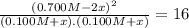
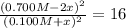
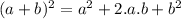
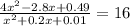
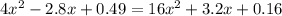
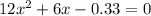
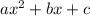 is:
is:![x_{1} =\frac{-b+\sqrt[]{4.a.c} }{2.a} \\x_{2} =\frac{-b-\sqrt[]{4.a.c} }{2.a}](/tpl/images/0281/3856/fdc5b.png)
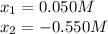
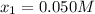
![[N_{2}] = 0.100 M + x = 0.150 M; [O_{2}] = 0.100 M + x = 0.150 M; [NO] = 0.700 M - 2x = 0.600 M](/tpl/images/0281/3856/a4921.png)
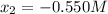
![[N_{2}] = 0.100 M + x = -0.450 M; [O_{2}] = 0.100 M + x = -0.450 M; [NO] = 0.700 M - 2x = 1.800 M](/tpl/images/0281/3856/d9901.png)
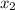 isn´t a possible answer because it results in negative equilibrium concentrations of the reagents. Then Case
isn´t a possible answer because it results in negative equilibrium concentrations of the reagents. Then Case 

