
Chemistry, 02.10.2019 03:00 bowmanari2154
Consider the nucleophilic addition reaction of 2‑butanone with excess propyl magnesiumbromide, made in situ by reacting 1‑bromopropane with metallic magnesium, to make 3‑methyl‑3‑hexanol. 2-butanone 1-bromopropane magnesium 3-methyl-3-hexanol =0.81 g/ml =1.35 g/ml =0.82 g/ml a reaction was performed in which 0.40 ml0.40 ml of 2‑butanone was reacted with an excess of propyl magnesiumbromide to make 0.38 g0.38 g of 3‑methyl‑3‑hexanol. calculate the theoretical yield and percent yield for this reaction.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 22.06.2019 14:30
How do temperature and salinity affect deepwater currents? as temperatures and salinity levels of water increase, the water rises to the surface where it creates currents as it moves to colder regions. they create changes in wind direction, moving denser water in the same direction as the wind and causing the deepwater circulation patterns found in the ocean. they equalize the forces on undersea currents caused by the coriolis effect as they replace more dense water with less dense water. they create density differences that cause dense deepwater currents to flow toward the equator where they displace less dense, warmer water above them.
Answers: 2

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2
You know the right answer?
Consider the nucleophilic addition reaction of 2‑butanone with excess propyl magnesiumbromide, made...
Questions




Mathematics, 29.03.2021 16:50

Mathematics, 29.03.2021 16:50


Mathematics, 29.03.2021 16:50

Mathematics, 29.03.2021 16:50

Computers and Technology, 29.03.2021 16:50


Biology, 29.03.2021 16:50




History, 29.03.2021 16:50


Chemistry, 29.03.2021 16:50



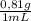 ×
×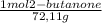 ×
×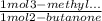 ×
×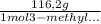 = 0,52 g
= 0,52 g

