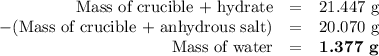Analo387: percent water in a hydrate
3. the mass of a crucible and a hydrated salt was found...

Chemistry, 02.10.2019 17:30 abdullaketbi71
Analo387: percent water in a hydrate
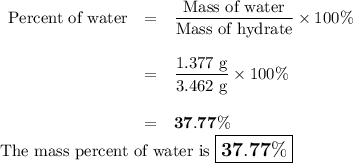
3. the mass of a crucible and a hydrated salt was found to be 21.447 g. the mass of the crucible and the
anhydrous salt was 20.070 g. the mass of the crucible was 17.985 g.
(1) calculate the mass of the hydrate heated.
(2) calculate the mass of water lost from the hydrate during heating.
(3) calculate the percent water in the hydrate.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. initial mass and yield sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 1

Chemistry, 21.06.2019 22:00
Your friend offers to show you an intrusive igneous rock. which of the following would you expect to see?
Answers: 1

Chemistry, 22.06.2019 01:30
(apex) when a cup of water is dropped, as the cup falls, the water in the cup falls out true or false?
Answers: 1

Chemistry, 22.06.2019 09:00
Scientific evidence tells us that the cause of earths four season is the tilt of earth as it revolves around the sun. the student is instructed to illustrate this information in a science notebook. how will the student illiterate winter in the northern hemisphere?
Answers: 3
You know the right answer?
Questions


Health, 04.07.2019 04:30




History, 04.07.2019 04:30

Mathematics, 04.07.2019 04:30


Mathematics, 04.07.2019 04:30



Mathematics, 04.07.2019 04:30





Computers and Technology, 04.07.2019 04:30


English, 04.07.2019 04:30