
Astudent is extracting caffeine from water with dichloromethane. the k value is 4.6. if the student starts with a total of 40 mg of caffeine in 2 ml of water and extracts once with 6 ml of nioromethane, how much caffeine will be in the dichloromethane extract? show all work.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 22.06.2019 17:30
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3

You know the right answer?
Astudent is extracting caffeine from water with dichloromethane. the k value is 4.6. if the student...
Questions


Mathematics, 20.10.2019 20:20

Mathematics, 20.10.2019 20:20


Mathematics, 20.10.2019 20:20



Mathematics, 20.10.2019 20:20

History, 20.10.2019 20:20




History, 20.10.2019 20:20

Social Studies, 20.10.2019 20:20

Social Studies, 20.10.2019 20:20

Computers and Technology, 20.10.2019 20:20

History, 20.10.2019 20:20



Mathematics, 20.10.2019 20:20

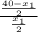
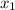 = 7.2 mg
= 7.2 mg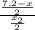
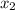 = 1.29 mg
= 1.29 mg
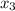 = 0.229 mg
= 0.229 mg

