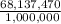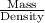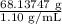Chemistry, 02.10.2019 20:00 ewalchloe5067920
Asolution used to chlorinate a home swimming pool contains 7% chlorine by mass. an ideal chlorine level for the pool is one part per million (1 ppm). (think of 1 ppm as being 1 g chlorine per million grams of water). if you assume densities of 1.10 g/ml for the chlorine solution and 1.00 g/ml for the swimming pool water, what volume of the chlorine solution, in liters, is required to produce a chlorine level of 1 ppm in an 18,000 gal swimming pool?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 12:00
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2
You know the right answer?
Asolution used to chlorinate a home swimming pool contains 7% chlorine by mass. an ideal chlorine le...
Questions


English, 19.05.2021 01:00


Mathematics, 19.05.2021 01:00

Mathematics, 19.05.2021 01:00


Physics, 19.05.2021 01:00

English, 19.05.2021 01:00


Chemistry, 19.05.2021 01:00

Computers and Technology, 19.05.2021 01:00

Mathematics, 19.05.2021 01:00

English, 19.05.2021 01:00





Mathematics, 19.05.2021 01:00


Biology, 19.05.2021 01:00