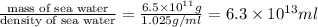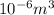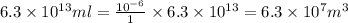Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 12:50
Use the standard enthalpies of formation for the reactants and products to solve for the δhrxn for the following reaction. (the δhf of c2h4 is 52.26 kj/mol, co2 is -393.509 kj/mol, and h2o is -241.818 kj.) c2h4 (g) + 3o2(g) 2co2 (g) + 2h2o(g) δhrxn = the reaction is .
Answers: 3


Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

You know the right answer?
Magnesium occurs in seawater to the extent of 1.4 g magnesium per kilogram of seawater. what volume...
Questions

Mathematics, 13.04.2021 20:00

Mathematics, 13.04.2021 20:00


Mathematics, 13.04.2021 20:00


Mathematics, 13.04.2021 20:00



Biology, 13.04.2021 20:00


Mathematics, 13.04.2021 20:00

Chemistry, 13.04.2021 20:00


Mathematics, 13.04.2021 20:00

Mathematics, 13.04.2021 20:00

SAT, 13.04.2021 20:00

Physics, 13.04.2021 20:00


Mathematics, 13.04.2021 20:00

Mathematics, 13.04.2021 20:00

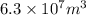
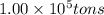
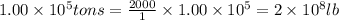

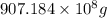 of magnesium is produced by =
of magnesium is produced by =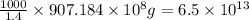 g of sea water
g of sea water