
Chemistry, 02.10.2019 20:00 belllerzz2678
How manymoles of each ion are present in 175 ml of 0.147 m fe2(so4)3?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
In a laboratory experiment, a fermenting aqueous solution of glucose and yeast produces carbon dioxide gas and ethanol. the solution was heated by burning natural gas in a bunsen burner to distill the ethanol that formed in the flask. during the distillation, the ethanol evaporated and then condensed in the receiving flask. the flame of the burner was kept too close to the bottom of the flask and some of the glucose decomposed into a black carbon deposit on the inside of the flask. during this experiment the following changes occurred. which of these changes involved a physical change and not a chemical change? check all that apply. 1-condensation of ethanol 2-evaporation of ethanol 3- formation of carbon dioxide gas from glucose burning of natural gas 4-formation of ethanol from glucose by yeast 5-formation of a carbon deposit inside the flask
Answers: 2

Chemistry, 22.06.2019 02:10
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2

Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1

You know the right answer?
How manymoles of each ion are present in 175 ml of 0.147 m fe2(so4)3?...
Questions

Business, 10.11.2020 01:00

Mathematics, 10.11.2020 01:00

Mathematics, 10.11.2020 01:00

English, 10.11.2020 01:00

History, 10.11.2020 01:00



Physics, 10.11.2020 01:00

Mathematics, 10.11.2020 01:00

Mathematics, 10.11.2020 01:00

Computers and Technology, 10.11.2020 01:00

Business, 10.11.2020 01:00

Mathematics, 10.11.2020 01:00



Mathematics, 10.11.2020 01:00


Mathematics, 10.11.2020 01:00

Mathematics, 10.11.2020 01:00

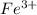 and 0.0257 moles of
and 0.0257 moles of 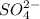
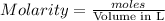
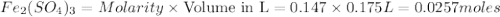
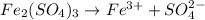
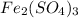 gives 1 mole of
gives 1 mole of 

