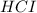Hydrocyanic acid, hcn, is a weak acid. (a) write the chemical equation for the dissociation of hcn in water. (b) identify the brønsted-lowry conjugate acid-base pairs in the equation above. (c) write the chemical equation for the reaction of hcn with naoh. (d) write the chemical equation for dissociation of nacn in water. (e) write the chemical equation for the reaction of nacn and hci.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 09:30
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3

Chemistry, 23.06.2019 00:30
What is calcium oxide+diphosphorus pentoxide--> calcium phosphate balanced
Answers: 1
You know the right answer?
Hydrocyanic acid, hcn, is a weak acid. (a) write the chemical equation for the dissociation of hcn i...
Questions



Physics, 10.10.2019 03:30

English, 10.10.2019 03:30

Mathematics, 10.10.2019 03:30


History, 10.10.2019 03:30


Mathematics, 10.10.2019 03:30




Mathematics, 10.10.2019 03:30


Business, 10.10.2019 03:30


Social Studies, 10.10.2019 03:30



Mathematics, 10.10.2019 03:30

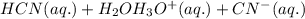
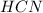 : acid
: acid 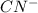 :conjugate base.
:conjugate base.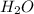 : base
: base 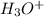 : conjugate acid.
: conjugate acid.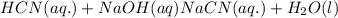
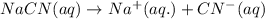
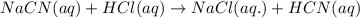
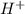 ions in their aqueous states.
ions in their aqueous states.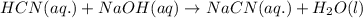
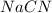 in water.
in water.