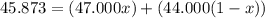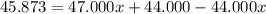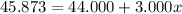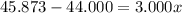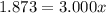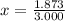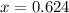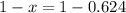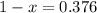Chemistry, 02.10.2019 20:10 BigGirlsTheBest
An element has only two naturally occurring isotopes. you know that isotope 1 has a mass of 44.000 amu and isotope 2 has a mass of 47.000 amu. if the average atomic mass is 45.873 amu, what are the natural abundances in percent for the two isotopes? 14.00m

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 07:00
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 07:40
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na,so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1

Chemistry, 22.06.2019 21:30
While in europe, if you drive 125 km per day, how much money would you spend on gas in one week if gas costs 1.10 euros per liter and your car’s gas mileage is 32.0 mi/gal? assume that 1 euro=1.26 dollars
Answers: 2
You know the right answer?
An element has only two naturally occurring isotopes. you know that isotope 1 has a mass of 44.000 a...
Questions

Mathematics, 03.02.2020 06:51

History, 03.02.2020 06:51


Mathematics, 03.02.2020 06:51

History, 03.02.2020 06:51


English, 03.02.2020 06:51

Mathematics, 03.02.2020 06:51


Mathematics, 03.02.2020 06:51


Mathematics, 03.02.2020 06:51



History, 03.02.2020 06:51

Chemistry, 03.02.2020 06:51




Mathematics, 03.02.2020 06:51