
Chemistry, 02.10.2019 20:10 21ghostrider21
Describe how you would prepare 100 ml of a 0.050 m sodium citrate tribasic buffer to a ph of 6. this method is accomplished by preparing a solution of a weak species base and then adding the appropriate strong species 0.5 m hcl to get to the desired ph. what amount of mass or volumes should you add?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

Chemistry, 22.06.2019 14:00
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1
You know the right answer?
Describe how you would prepare 100 ml of a 0.050 m sodium citrate tribasic buffer to a ph of 6. this...
Questions


English, 26.08.2019 02:30

Mathematics, 26.08.2019 02:30



Social Studies, 26.08.2019 02:30

Social Studies, 26.08.2019 02:30


Social Studies, 26.08.2019 02:30


History, 26.08.2019 02:30

Mathematics, 26.08.2019 02:30


Chemistry, 26.08.2019 02:30

English, 26.08.2019 02:30



Advanced Placement (AP), 26.08.2019 02:30

Law, 26.08.2019 02:30

![\frac{[A^{-}]}{[HA]}](/tpl/images/0284/0047/5980f.png)
![\frac{[CitrateTribasic]}{[CitrateDibasic]}](/tpl/images/0284/0047/f66e1.png)
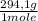 = 1,4705 g of sodium citrate tribasic dihydrate -commercial reactant-
= 1,4705 g of sodium citrate tribasic dihydrate -commercial reactant-

