
Chemistry, 02.10.2019 20:20 rebecca7415
A585−g piece of copper tubing is heated to 89.5°c and placed in an insulated vessel containing 159 g of water at 22.8°c. assuming no loss of water and a heat capacity for the vessel of 10.0 j/°c, what is the final temperature of the system (c of copper = 0.387 j/g·°c)?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:40
Modern pennies are composed of zinc coated with copper. a student determines the mass of a penny to be 2.482 g and then makes several scratches in the copper coaling (to expose the underlying zinc). the student puts the scratched penny in hydrochloric acid, where the following reaction occurs between the zinc and the hcl (the copper remains undissolved): zn(s) + 2 hcl(aq) → h2(g) + zncl(aq)the student collects the hydrogen produced over water at 25 °c. the collected gas occupies a volume of 0.899 l at a total pressure of 79 j mmhg. calculate the percent zinc (by mass) in the penny. (assume that all the zn in the penny dissolves.)
Answers: 1

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1

Chemistry, 23.06.2019 01:00
What is the chemical name of the compound ti2o3? use the list of polyatomic ions and the periodic table to you answer.
Answers: 1
You know the right answer?
A585−g piece of copper tubing is heated to 89.5°c and placed in an insulated vessel containing 159 g...
Questions

History, 21.09.2019 00:30

Physics, 21.09.2019 00:30

Physics, 21.09.2019 00:30


History, 21.09.2019 00:30

Business, 21.09.2019 00:30

Computers and Technology, 21.09.2019 00:30



Social Studies, 21.09.2019 00:30

Mathematics, 21.09.2019 00:30




Mathematics, 21.09.2019 00:30

Mathematics, 21.09.2019 00:30

Mathematics, 21.09.2019 00:30

Mathematics, 21.09.2019 00:30


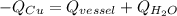 (1)
(1)

