
Chemistry, 02.10.2019 21:00 sandeebassett3
Common brass is a copper and zinc alloy containing 37.0% zinc by mass and having a density of 8.48 g/cm3. a fitting composed of common brass has a total volume of 122.5 cm3 .
how many atoms of copper does the fitting contain?
how many atoms of zinc does the fitting contain?

Answers: 3


Another question on Chemistry


Chemistry, 23.06.2019 06:30
Consider the heating curve of h2o and line segments a, b, and c. several changes are taking place at a, b, and c. all but one would be an appropriate description as e move through segments a, b and then c.
Answers: 3

Chemistry, 23.06.2019 13:00
Using the periodic table complete the table to describe each atom type in your answers
Answers: 1

Chemistry, 23.06.2019 13:20
Which nuclide is most likely to be radioactive and synthetic 24/12 mg237/93mg195/78mg230/84mg
Answers: 1
You know the right answer?
Common brass is a copper and zinc alloy containing 37.0% zinc by mass and having a density of 8.48 g...
Questions


History, 23.07.2019 02:30

Health, 23.07.2019 02:30


Chemistry, 23.07.2019 02:30


Chemistry, 23.07.2019 02:30



Biology, 23.07.2019 02:30

Social Studies, 23.07.2019 02:30




Computers and Technology, 23.07.2019 02:30

Mathematics, 23.07.2019 02:30


Mathematics, 23.07.2019 02:40

Mathematics, 23.07.2019 02:40

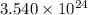

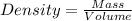
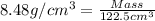
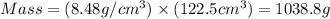
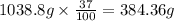
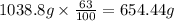
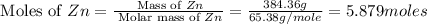
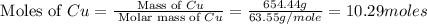
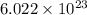 number of copper atoms
number of copper atoms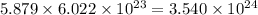 number of copper atoms
number of copper atoms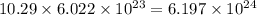 number of zinc atoms
number of zinc atoms

