
Chemistry, 02.10.2019 21:00 gorbyalexis
Calculate the equilibrium concentrations of n2o4 and no2 at 25 ∘c in a vessel that contains an initial n2o4 concentration of 0.0654 m . the equilibrium constant kc for the reaction n2o4(g)⇌2no2(g) is 4.64×10−3 at 25 ∘c.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
100 points answer quick the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1


Chemistry, 23.06.2019 02:00
When an experimenter draws a conclusion that he assumes will apply to all situations set up similarly to his test situation, even though he cannot possibly have examined all possible test scenarios, the experimenter is using deductive reasoning inductive reasoning abductive reasoning subjective reasoning
Answers: 1

Chemistry, 23.06.2019 02:30
Calculate the ph at the equivalence point for the titration of a solution containing 150.0 mg of ethylamine (c2h5nh2) with 0.1000 m hcl solution. the volume of the solution at the equivalence point is 250.0 ml. kb forethylamine is 4.7 × 10−4 .
Answers: 2
You know the right answer?
Calculate the equilibrium concentrations of n2o4 and no2 at 25 ∘c in a vessel that contains an initi...
Questions


History, 16.10.2020 04:01


Mathematics, 16.10.2020 04:01


Biology, 16.10.2020 04:01

History, 16.10.2020 04:01

Physics, 16.10.2020 04:01


Biology, 16.10.2020 04:01


Mathematics, 16.10.2020 04:01

Business, 16.10.2020 04:01

Mathematics, 16.10.2020 04:01

Biology, 16.10.2020 04:01

Mathematics, 16.10.2020 04:01



Mathematics, 16.10.2020 04:01

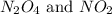 are 0.0164 M and 0.0572 M
are 0.0164 M and 0.0572 M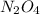 = 0.0654 M
= 0.0654 M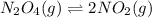
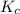 for above reaction follows:
for above reaction follows:![K_c=\frac{[NO_2]_{eq}^2}{[N_2O_4]_{eq}}](/tpl/images/0284/1377/77dc1.png)
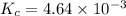
![[NO_2]_{eq}=2x](/tpl/images/0284/1377/0bbcc.png)
![[N_2O_4]_{eq}=0.0654-x](/tpl/images/0284/1377/db879.png)
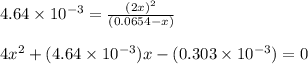
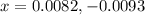
![[NO_2]_{eq}=2x=2(0.0082)=0.0164M](/tpl/images/0284/1377/53c5f.png)
![[N_2O_4]_{eq}=0.0654-x=(0.0654-0.0082)=0.0572M](/tpl/images/0284/1377/125d4.png)



