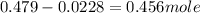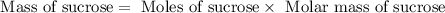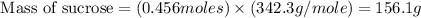The hydrolysis of sucrose (c12h22011) into glucose and fructose in acidic solution is a first-order reaction with a rate constant of 1.8 x 10-45-1 at 25°c. determine the mass (g) of sucrose that is consumed when 2.15 l of a 0.223 m sucrose solution is allowed to react for 282 minutes. enter your answer as an integer. previous next not saved submit qu.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2

Chemistry, 22.06.2019 17:30
I'm learning about the periodic tables and what each subject's configuration is. for example, hydrogen is 1s^1, but i don't understand how you get that. can someone me understand how to figure out how to figure this out? sorry if the question makes no sense, but it would really a lot if you could me understand! you so much if you can!
Answers: 1

You know the right answer?
The hydrolysis of sucrose (c12h22011) into glucose and fructose in acidic solution is a first-order...
Questions



Mathematics, 18.06.2021 02:00

Social Studies, 18.06.2021 02:00



Mathematics, 18.06.2021 02:00




English, 18.06.2021 02:00



Mathematics, 18.06.2021 02:00

Social Studies, 18.06.2021 02:00

History, 18.06.2021 02:00

English, 18.06.2021 02:00

History, 18.06.2021 02:00

Biology, 18.06.2021 02:00

Mathematics, 18.06.2021 02:00

![[C_t]=[C_o]e^{-kt}](/tpl/images/0284/1096/86a58.png)
![[C_t]](/tpl/images/0284/1096/81c01.png) = concentration of sucrose at time 't'
= concentration of sucrose at time 't'![[C_o]](/tpl/images/0284/1096/61f0a.png) = concentration of sucrose at time '0' = 0.223 M
= concentration of sucrose at time '0' = 0.223 M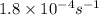
![[C_t]=(0.223)\times e^{-(1.8\times 10^{-4})\times (16920)}](/tpl/images/0284/1096/fccf3.png)
![[C_t]=0.0106M](/tpl/images/0284/1096/bb844.png)
![n_o=[C_o]\times V=0.223M\times 2.15L=0.479moles](/tpl/images/0284/1096/1c118.png)
![n_t=[C_t]\times V=0.0106M\times 2.15L=0.0228moles](/tpl/images/0284/1096/96015.png)