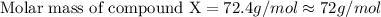When 5.42 g of a certain molecular compound x are dissolved in 80.0 g of formamide (nh, coh), the freezing point of the solution is measured to be -1.4 °c. calculate the molar mass of x. if you need any additional information on formamide, use only what you find in the aleks data resource. also, be sure your answer has a unit symbol, and is rounded to 2 significant digits. one x 6 ?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1

Chemistry, 22.06.2019 23:00
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1

Chemistry, 23.06.2019 00:30
Nuclear decay is the spontaneous decay of one element into a. an x-ray b. a ray of light c. another element
Answers: 1

Chemistry, 23.06.2019 01:30
Magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. trial 1: trial 2: data mass of empty crucible with lid trial 1: 26.688 trial 2: 26.681 mass of mg metal, crucible, and lid trial 1: 26.994 trial: 2 26.985 mass of mgo, crucible, and lid trial 1: 27.188 trial 2: 27.180
Answers: 1
You know the right answer?
When 5.42 g of a certain molecular compound x are dissolved in 80.0 g of formamide (nh, coh), the fr...
Questions

Arts, 08.12.2020 01:10


Mathematics, 08.12.2020 01:10

Mathematics, 08.12.2020 01:10



Mathematics, 08.12.2020 01:10




History, 08.12.2020 01:10

Physics, 08.12.2020 01:10

English, 08.12.2020 01:10




Spanish, 08.12.2020 01:10



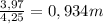
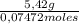 = 73 g/mol
= 73 g/mol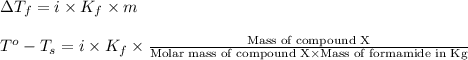
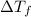 = change in freezing point
= change in freezing point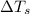 = freezing point of solution =
= freezing point of solution = 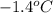
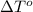 = freezing point of formamide =
= freezing point of formamide = 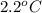
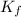 = freezing point constant for formamide =
= freezing point constant for formamide = 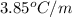
![[2.2-(-1.4)]^oC=1\times (3.85^oC/m)\times \frac{5.42g}{\text{Molar mass of compound X}\times 0.080kg}](/tpl/images/0284/0592/99dda.png)