

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Consider the point on the plot where 10.0 g of naoh have been added. what amount of naoh, in moles, has been added? 0.308 mol fecl3 initially present
Answers: 1

Chemistry, 21.06.2019 22:30
Ibeg i need 20. a reaction produces 4.93 l of oxygen, but was supposed to produce 1 mol of oxygen. what is the percent yield?
Answers: 1

Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1

Chemistry, 22.06.2019 19:20
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3
You know the right answer?
When nonpolar solutes are placed in water, the hydrogen bonding network of water is disrupted, and t...
Questions

Mathematics, 14.12.2020 08:20



History, 14.12.2020 08:20

Computers and Technology, 14.12.2020 08:20

History, 14.12.2020 08:20

Chemistry, 14.12.2020 08:20

Mathematics, 14.12.2020 08:20

Mathematics, 14.12.2020 08:20





Mathematics, 14.12.2020 08:20




Mathematics, 14.12.2020 08:30

Mathematics, 14.12.2020 08:30

History, 14.12.2020 08:30

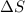 is positive when randomness increases and
is positive when randomness increases and 

