
Chemistry, 02.10.2019 21:10 alexandroperez13
If you used 0.75 grams of p-aminophenol and got 0.74 grams of acetaminophen, what is the percent yield?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. initial mass and yield sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 1

Chemistry, 22.06.2019 04:30
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3

Chemistry, 22.06.2019 08:30
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

You know the right answer?
If you used 0.75 grams of p-aminophenol and got 0.74 grams of acetaminophen, what is the percent yie...
Questions

Mathematics, 21.02.2021 18:50

Chemistry, 21.02.2021 18:50


Social Studies, 21.02.2021 18:50

Advanced Placement (AP), 21.02.2021 18:50

Mathematics, 21.02.2021 18:50

Mathematics, 21.02.2021 18:50

Mathematics, 21.02.2021 18:50



Mathematics, 21.02.2021 18:50




History, 21.02.2021 18:50


Biology, 21.02.2021 18:50


English, 21.02.2021 18:50

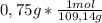 = 6,87x10⁻³ moles
= 6,87x10⁻³ moles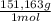 = 1,04 grams
= 1,04 grams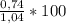 = 71,2%
= 71,2%

