
Butylated hydroxytoluene (bht) is used as an antioxidant in processed foods. (it prevents fats and oils from becoming rancid.) a solution of 2.500 g of bht in 100.0 g of benzene had a freezing point of 4.880 oc. what is molecular mass of bht? . kf for benzene is 5.065 oc/m

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:10
Abullet found at a crime scene may be used as evidence in a trial if the percentage of metals match to the composition of metals in a bullet from the suspect's ammunition. a forensic scientist's analysis of the bullet shows that it contains 11.9 g of lead, 0.5 g of tin, and 0.8 b of antimony. what is the percentage of lead metal in the bullet? express your answers to the one's place.
Answers: 2

Chemistry, 22.06.2019 08:30
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1

Chemistry, 22.06.2019 10:30
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2

You know the right answer?
Butylated hydroxytoluene (bht) is used as an antioxidant in processed foods. (it prevents fats and o...
Questions

Physics, 10.11.2020 20:40

Chemistry, 10.11.2020 20:40

Mathematics, 10.11.2020 20:40


Mathematics, 10.11.2020 20:40



Computers and Technology, 10.11.2020 20:40


Law, 10.11.2020 20:40



Biology, 10.11.2020 20:40

History, 10.11.2020 20:40

Mathematics, 10.11.2020 20:40

Mathematics, 10.11.2020 20:40

English, 10.11.2020 20:40



Law, 10.11.2020 20:40

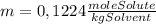
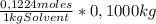 = 0,01224 moles of solute ≡ BHT
= 0,01224 moles of solute ≡ BHT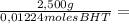 204,2 g/mol
204,2 g/mol

