
Chemistry, 02.10.2019 22:00 TropicalFan
Certain atoms emit photons of light with an energy of 3.820 ✕ 10−19 j. calculate the frequency (in hz) and wavelength (in nm) of one of these photons.
frequency hz
and
wavelength nm
what is the total energy (in kj) in 1 mole of these photons?
kj

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Y=‐1x + 7 if y has a value of ‐24 what is the value of x?
Answers: 1

Chemistry, 22.06.2019 09:20
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 23.06.2019 03:00
In november 1987, a massive iceberg broke loose from the antartic ice mass and floated free in the ocean. the chunk of ice was estimated to be 98 mi long, 25 mi wide, and 750 ft thick. a typical backyard swimming pool contains about 24,000 gallons of water. how many of these pools could you fill from the water in this iceberg? (assume the iceberg is a rectangular solid of the above dimensions and consists of water only). express answer in scientific notation.
Answers: 1
You know the right answer?
Certain atoms emit photons of light with an energy of 3.820 ✕ 10−19 j. calculate the frequency (in h...
Questions



English, 05.10.2019 18:30

Health, 05.10.2019 18:30

Mathematics, 05.10.2019 18:30

Geography, 05.10.2019 18:30


Mathematics, 05.10.2019 18:30

Mathematics, 05.10.2019 18:30


Mathematics, 05.10.2019 18:30


Biology, 05.10.2019 18:30





English, 05.10.2019 18:30


English, 05.10.2019 18:30

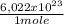 = 230040 J/mole ≡ 230,0 kJ/mole
= 230040 J/mole ≡ 230,0 kJ/mole

