
Chemistry, 02.10.2019 22:00 HELPPPPPfawfds
You need to prepare 0.2 liters of a 0.1 m solution of a phosphate buffer with a ph of 7.2. if you have solid k2hpo4 (fw=136.09) and 6m hcl or 6 m naoh describe how you would prepare this buffer. note: pka values for phosphoric acid are 2.15, 6.86, and 12.35

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:00
The twister and runaway train are two coasters at the same amusement park. both coasters start at the same height. the coaster for the twister is twice the mass of the coaster for the runaway train. which roller coaster has greater gravitational potential energy at the start of the ride?
Answers: 1

Chemistry, 22.06.2019 20:20
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3

Chemistry, 23.06.2019 03:20
High-pressure liquid chromatography (hplc) is a method used in chemistry and biochemistry to purify chemical substances. the pressures used in this procedure range from around 500 kilopascals (500,000 pa) to about 60,000 kpa (60,000,000 pa). it is often convenient to know the pressure in torr. if an hplc procedure is running at a pressure of 1.03×108 pa , what is its running pressure in torr?
Answers: 3

You know the right answer?
You need to prepare 0.2 liters of a 0.1 m solution of a phosphate buffer with a ph of 7.2. if you ha...
Questions







History, 20.04.2020 19:56

Mathematics, 20.04.2020 19:56



Mathematics, 20.04.2020 19:56



Mathematics, 20.04.2020 19:56


History, 20.04.2020 19:56

Mathematics, 20.04.2020 19:56



![\frac{[HPO_{4}^{2-}]}{[H_{2}PO_{4}^-]}](/tpl/images/0284/2917/12fd1.png)
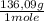 = 2,722 g
= 2,722 g

