Identifying the conjugate base which is the conjugate base in each of the pairs below?
rcooh,...


Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 22.06.2019 13:00
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3

Chemistry, 22.06.2019 20:00
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1
You know the right answer?
Questions

Mathematics, 07.05.2020 06:00

Mathematics, 07.05.2020 06:00


History, 07.05.2020 06:00







Advanced Placement (AP), 07.05.2020 06:00

Biology, 07.05.2020 06:00









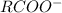 that is the conjugate base; and the water gains the H+ and become the conjugate acid, that is:
that is the conjugate base; and the water gains the H+ and become the conjugate acid, that is: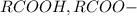
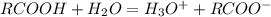
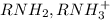
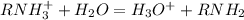
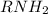 is the conjugate base
is the conjugate base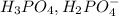
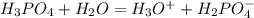
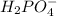 is the conjugate base
is the conjugate base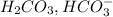
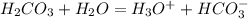
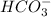 is the conjugate base.
is the conjugate base.

