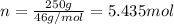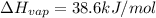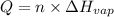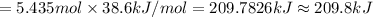Chemistry, 02.10.2019 22:00 adriandehoyos1p3hpwc
Ethanol, ch.0, is common beverage alcohol. at its boiling point of 78.5 °c, the enthalpy of vaporization of ethanol is 38.6 kj/mol. how much heat is required to vaporize 250 g of ethanol at 78.5 °c? ans = 209.8 kj

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Find the empirical formula of each of the following compounds. given mass or for each element in a sample of the compound 3,611 g ca; 6.389 g c1
Answers: 1

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 2

Chemistry, 22.06.2019 12:00
Most materials are not magnetic because their magnetism has worn off. their magnetic domains are arranged randomly. they lack magnetic fields. earth’s heat has destroyed their magnetism.
Answers: 1

Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1
You know the right answer?
Ethanol, ch.0, is common beverage alcohol. at its boiling point of 78.5 °c, the enthalpy of vaporiza...
Questions

Mathematics, 11.12.2020 05:10

History, 11.12.2020 05:10

Social Studies, 11.12.2020 05:10


Mathematics, 11.12.2020 05:10

History, 11.12.2020 05:10

Mathematics, 11.12.2020 05:10


Mathematics, 11.12.2020 05:10

Mathematics, 11.12.2020 05:10




Mathematics, 11.12.2020 05:10

Mathematics, 11.12.2020 05:10


Mathematics, 11.12.2020 05:10