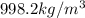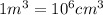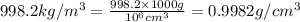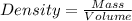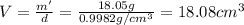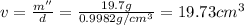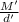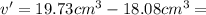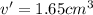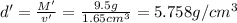Chemistry, 02.10.2019 21:30 timjape3g3z
Apycnometer is a precisely weighted vessel that is used for highly accurate density determinations. suppose that a pycnometer has a mass of 27.60 g when it is empty and has a mass of 45.65 g when it is completely filled with water at 20 °c. a 9.5 g metal ingot is placed in the pycnometer. when it is then filled with water at 20 °c, the total mass is 56.83 g. if the density of water at 20 °c is 998.2 kg/m3, what is the density of the metal ingot in grams per cubic centimeter.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
What layer of the atmosphere is directly above the troposphere?
Answers: 1

Chemistry, 22.06.2019 10:00
Drug abuse will not lead to physical and psychological dependence. true or false ?
Answers: 2

Chemistry, 22.06.2019 21:20
The organs inside the body and how they function together
Answers: 3

Chemistry, 22.06.2019 23:30
The comparison of the number of atoms in a copper coin the size of a penny with the number of people on earth is made to illustrate which of the following? a. that atoms are indivisible b. that atoms are very small c. that atoms are very large d. that in a copper penny, there is one atom for every person on earth
Answers: 1
You know the right answer?
Apycnometer is a precisely weighted vessel that is used for highly accurate density determinations....
Questions


Arts, 01.01.2022 07:10

Mathematics, 01.01.2022 07:10

Social Studies, 01.01.2022 07:10




Social Studies, 01.01.2022 07:20


Physics, 01.01.2022 07:20

Computers and Technology, 01.01.2022 07:20





Mathematics, 01.01.2022 07:30

Business, 01.01.2022 07:30