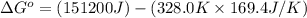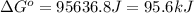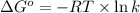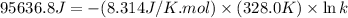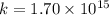For the reaction fe3o4(s) + 4h2(g) --> 3fe(s) + 4h2o(g)
h° = 151.2 kj and s° = 169.4...

Chemistry, 02.10.2019 21:30 bluebabyyy
For the reaction fe3o4(s) + 4h2(g) --> 3fe(s) + 4h2o(g)
h° = 151.2 kj and s° = 169.4 j/k
the equilibrium constant for this reaction at 328.0 k is

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3

Chemistry, 22.06.2019 21:00
As we move from left to right across the periodic table, what is the general trend? a) atomic radii increase. b) electronegavitiy decreases. c) nuclear shielding increases. d) metallic character decreases.
Answers: 1
You know the right answer?
Questions

History, 30.09.2019 02:10



History, 30.09.2019 02:10


Mathematics, 30.09.2019 02:10

Mathematics, 30.09.2019 02:10



Chemistry, 30.09.2019 02:10

Mathematics, 30.09.2019 02:10

Mathematics, 30.09.2019 02:10


Physics, 30.09.2019 02:10


Mathematics, 30.09.2019 02:10

English, 30.09.2019 02:10



History, 30.09.2019 02:10


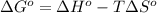
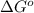 = standard Gibbs free energy = ?
= standard Gibbs free energy = ?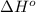 = standard enthalpy = 151.2 kJ = 151200 J
= standard enthalpy = 151.2 kJ = 151200 J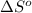 = standard entropy = 169.4 J/K
= standard entropy = 169.4 J/K