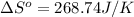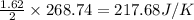Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Two nitro no2 groups are chemically bonded to a patch of surface. they can't move to another location on the surface, but they can rotate (see sketch at right). it turns out that the amount of rotational kinetic energy each no2 group can have is required to be a multiple of ε, where =ε×1.010−24 j. in other words, each no2 group could have ε of rotational kinetic energy, or 2ε, or 3ε, and so forth — but it cannot have just any old amount of rotational kinetic energy. suppose the total rotational kinetic energy in this system is initially known to be 32ε. then, some heat is removed from the system, and the total rotational kinetic energy falls to 18ε. calculate the change in entropy. round your answer to 3 significant digits, and be sure it has the correct unit symbol.
Answers: 2

Chemistry, 22.06.2019 18:30
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1

Chemistry, 22.06.2019 23:30
How many grams of ammonia would be produced by the decomposition of 16.93 mlof hydrazine? (the density of hydrazine is 1.021g/ml)
Answers: 3

Chemistry, 23.06.2019 06:00
•what conclusions can you make about the relationship between the volume of a gas and its temperature? • what conclusions can you make about the relationship between the volume of a gas and its pressure? • what possible variables have you not accounted for? as you did the procedures, is it possible that the atmospheric pressure may have changed? if it did change over the course of your experiment, then how would your results have been affected?
Answers: 3
You know the right answer?
Consider the reaction: 2brf3(g) --> br2(g) + 3f2(g)
using standard absolute entropi...
using standard absolute entropi...
Questions


Chemistry, 21.09.2019 02:20

History, 21.09.2019 02:20


Biology, 21.09.2019 02:20




Mathematics, 21.09.2019 02:20

Mathematics, 21.09.2019 02:20


Mathematics, 21.09.2019 02:20

Computers and Technology, 21.09.2019 02:20

Mathematics, 21.09.2019 02:20

Mathematics, 21.09.2019 02:20

Mathematics, 21.09.2019 02:20

Mathematics, 21.09.2019 02:20

Mathematics, 21.09.2019 02:20


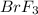 reacts at standard condition is 217.68 J/K
reacts at standard condition is 217.68 J/K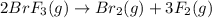
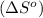 is:
is: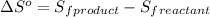
![\Delta S^o=[n_{Br_2}\times \Delta S_f^0_{(Br_2)}+n_{F_2}\times \Delta S_f^0_{(F_2)}]-[n_{BrF_3}\times \Delta S_f^0_{(BrF_3)}]](/tpl/images/0284/2284/c6a07.png)
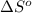 = entropy change of reaction = ?
= entropy change of reaction = ?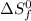 = standard entropy of formation
= standard entropy of formation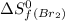 = 245.463 J/mol.K
= 245.463 J/mol.K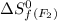 = 202.78 J/mol.K
= 202.78 J/mol.K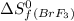 = 292.53 J/mol.K
= 292.53 J/mol.K![\Delta S^o=[1mole\times (245.463J/K.mole)+3mole\times (202.78J/K.mole)}]-[2mole\times (292.53J/K.mole)]](/tpl/images/0284/2284/4a0f6.png)