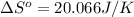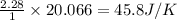Chemistry, 02.10.2019 21:30 dontworry48
Consider the reaction: h2(g) + cl2(g) --> 2hcl(g)
using standard absolute entropies at 298k, calculate the entropy change for the system when 2.28 moles of h2(g)react at standard conditions.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible? a. attractive forces between gas particles are negligible because the particles of an ideal gas are moving so quickly. b. collisions between gas particles are elastic; there is no net gain or loss of kinetic energy. c. gases consist of a large number of small particles, with a lot of space between the particles. d. gas particles are in constant, random motion, and higher kinetic energy means faster movement.
Answers: 1

Chemistry, 22.06.2019 10:00
Miner's coal distributors does not mine coal itself, nor does it even store or handle the coal. instead, miner's solicits orders for low sulfur coal from other firms, then purchases the required amount from suppliers and directs them to ship the coal to its customers. what is miner's
Answers: 1

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

You know the right answer?
Consider the reaction: h2(g) + cl2(g) --> 2hcl(g)
using standard absolute entropies...
using standard absolute entropies...
Questions

Advanced Placement (AP), 26.10.2020 22:50




History, 26.10.2020 22:50


Advanced Placement (AP), 26.10.2020 22:50

Mathematics, 26.10.2020 22:50



Mathematics, 26.10.2020 22:50


Mathematics, 26.10.2020 22:50





History, 26.10.2020 23:00


Mathematics, 26.10.2020 23:00

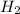 reacts at standard condition is 45.8 J/K
reacts at standard condition is 45.8 J/K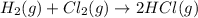
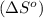 is:
is: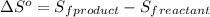
![\Delta S^o=[n_{HCl}\times \Delta S_f^0_{(HCl)}]-[n_{H_2}\times \Delta S_f^0_{(H_2)}+n_{Cl_2}\times \Delta S_f^0_{(Cl_2)}]](/tpl/images/0284/2286/d7d45.png)
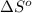 = entropy change of reaction = ?
= entropy change of reaction = ?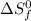 = standard entropy of formation
= standard entropy of formation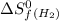 = 130.684 J/mol.K
= 130.684 J/mol.K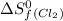 = 223.066 J/mol.K
= 223.066 J/mol.K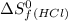 = 186.908 J/mol.K
= 186.908 J/mol.K![\Delta S^o=[2mole\times (186.908J/K.mole)]-[1mole\times (130.684J/K.mole)+1mole\times (223.066J/K.mole)}]](/tpl/images/0284/2286/55b6e.png)