

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
1. combine iron and copper (ii) sulfate solution. (hint: iron will form the iron (iii) ion) fe + cuso4 → 2. combine lead (ii) nitrate and potassium iodide solutions. pb(no3)2+ kl → 3. combine magnesium metal and hydrochloric acid solution. mg + hcl → 4. electrolysis (splitting) of water. h2o → 5. burning magnesium. mg + o2 →
Answers: 3

Chemistry, 22.06.2019 06:40
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3

Chemistry, 22.06.2019 11:30
If we compare and contrast electromagnetic waves with sound waves, all but one statement is true. that is a) sound waves require a medium to travel while electromagnetic waves do not. b) electromagnetic waves can travel through the vacuum of space while sound waves cannot. c) electromagnetic waves must have a medium in which to travel, but sound waves can travel anywhere. eliminate d) sound waves must bounce off of matter in order to travel while electromagnetic waves do not require matter to be present.
Answers: 3

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1
You know the right answer?
For a certain chemical reaction, the standard gibbs free energy of reaction at 10.0 °c is 149. kj. c...
Questions

Social Studies, 25.11.2020 22:30



Mathematics, 25.11.2020 22:30

Chemistry, 25.11.2020 22:30

Mathematics, 25.11.2020 22:30


Mathematics, 25.11.2020 22:30


History, 25.11.2020 22:30


Mathematics, 25.11.2020 22:30



Arts, 25.11.2020 22:30

English, 25.11.2020 22:30




History, 25.11.2020 22:30


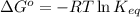
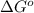 = standard Gibbs free energy = 149. kJ/mol = 149000 J/mol (Conversion factor: 1 kJ = 1000 J )
= standard Gibbs free energy = 149. kJ/mol = 149000 J/mol (Conversion factor: 1 kJ = 1000 J )
![15^oC=[273+15]K=283K](/tpl/images/0284/3257/82fe2.png)
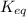 = equilibrium constant at 10°C = ?
= equilibrium constant at 10°C = ?


