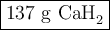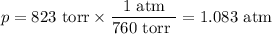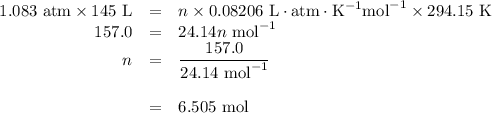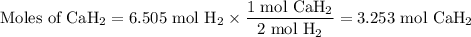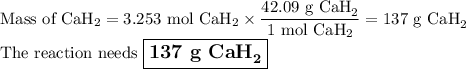Calcium hydride, cah2, reacts with water to form hydrogen gas:
cah2(s)+2h2o(l)→ca(oh)2(aq)+2...

Calcium hydride, cah2, reacts with water to form hydrogen gas:
cah2(s)+2h2o(l)→ca(oh)2(aq)+2h2(g)
this reaction is sometimes used to inflate life rafts, weather balloons, and the like, where a simple, compact means of generating h2 is desired.
how many grams of cah2 are needed to generate 145 l of h2 gas if the pressure of h2 is 823 torr at 21 ∘c?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Iwll give extra points to who gets this for ! what type of reaction is this? ?
Answers: 2

Chemistry, 21.06.2019 22:30
Imagine that you’re getting ready to move to a new city. when people move, they are influenced by push factors and pull factors, and you have many reasons for your move. which of the following factors is an example of a pull factor? a. wanting to move because you’ve found a great new school somewhere new b. needing to move because there are not enough resources in your old hometown c. being forced to move because your old home is gone d. having to move because there are no jobs in your current hometown
Answers: 1

Chemistry, 22.06.2019 02:30
You have a sample of a gas that occupies a volume of 17ml at -111 degrees celsius. what volume does the sample occupy at 88 degrees celsius? show all work asap
Answers: 3

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2
You know the right answer?
Questions










Computers and Technology, 02.03.2020 17:13

Computers and Technology, 02.03.2020 17:14