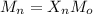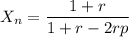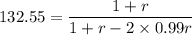Calculate the feed ratio of adipic acid and hexamethylene diamine that should be employed to obtain a polyamide of approximately 15,000 molecular weight at 99.5% conversion. what is the identity of the end groups of this product? do the same calculation for a 19,000-molecular-weight polymer.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:40
It is important to wear proper protective equipment in lab even when not actively performing experiments because accidents can affect any researcher, even one not working on an experiment. select the best answer from the choices provided
Answers: 3

Chemistry, 22.06.2019 07:30
In the particles are arranged in a regular, repeating pattern. a)a crystalline liquid b)a crystalline solid c)all gases d)all solids
Answers: 2

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1
You know the right answer?
Calculate the feed ratio of adipic acid and hexamethylene diamine that should be employed to obtain...
Questions

Mathematics, 20.11.2020 01:40





Geography, 20.11.2020 01:40

Geography, 20.11.2020 01:40


Mathematics, 20.11.2020 01:40


History, 20.11.2020 01:40


Mathematics, 20.11.2020 01:40

Spanish, 20.11.2020 01:40

English, 20.11.2020 01:40

English, 20.11.2020 01:40