Chemistry, 03.10.2019 05:00 genyjoannerubiera
18.1-1. diffusion of methane through helium. a gas of ch4 and he is contained in a tube at 101.32 kpa pressure and 298 k. at one point, the partial pressure of methane is pa1 = 60.79 kpa, and at a point 0.02 m distance away, pa2 = 20.26 kpa. if the total pressure is constant throughout the tube, calculate the flux of ch4 (methane) at steady state for equimolar counterdiffusion.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1

Chemistry, 23.06.2019 00:10
Apropane torch is lit inside a hot air balloon during preflight preparations to inflate the balloon. which condition of the gas remains constant
Answers: 2

Chemistry, 23.06.2019 02:00
Butane gas reacts with oxygen gas to give carbon dioxide gas and water vapor (gas). if you mix butane and oxygen in the correct stoichiometric ratio, and if the total pressure of the mixture is 390 mmhg, what is the pressure (in mmhg) of water vapor after the reaction is completed (temperature and volume do not change).
Answers: 2

You know the right answer?
18.1-1. diffusion of methane through helium. a gas of ch4 and he is contained in a tube at 101.32 kp...
Questions



Mathematics, 27.08.2020 02:01

Computers and Technology, 27.08.2020 02:01



English, 27.08.2020 02:01



Mathematics, 27.08.2020 02:01



History, 27.08.2020 02:01



Social Studies, 27.08.2020 02:01


History, 27.08.2020 02:01


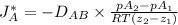
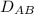 = 0.675 × 10⁻⁴ m²/s (for He-CH4 at 101.32 kPa and 298 K)
= 0.675 × 10⁻⁴ m²/s (for He-CH4 at 101.32 kPa and 298 K)