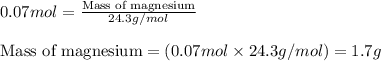Magnesium (used in the manufacture of light alloys) reacts with iron(iii) chloride to form magnesium chloride and iron. 3mg(s) + 2fecl₃(s) → 3mgcl₂(s) + 2fe(s)a mixture of 41.0 g of magnesium ( = 24.31 g/mol) and 175 g of iron(iii) chloride ( = 162.2 g/mol) is allowed to react. identify the limiting reactant and determine the mass of the excess reactant present in the vessel when the reaction is complete. a) limiting reactant is mg: 67g of fecl₃ remains. b) limiting reactant is mg: 134 g fecl₃ remains. c) limiting reactant is mg: 104 g fecl₃ remains. d) limiting reactant is fecl₃: 1.7 g of mg remans. e) limiting reactant is fecl₃: 87.2 g of mg remains.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 19:30
What is the common name for the compound shown here? enter the common name of the compound shown?
Answers: 2

Chemistry, 22.06.2019 21:20
Juju.01) 5 geologic events a group of students designed an experiment in an ice rink to represent the solar system. the steps of the experiment are listed below. 5: geologic events 1. choose a student to represent the sun. the planets are represented by two tennis balls. 2. ask the student to hold the tennis balls in each palm and spin on the ice with arms stretched out. 3. ask the student to draw in the arms after about 10 spins. 4. observe the student's arms rotate faster when they are closer to the body. 05 geologic events enors) the experiment most likely demonstrates that (2 points) 07 discussion-based sessment/module planets exert gravitational force on the sun the sun exerts gravitational force on the planets 3.07 discussion-based ssessment speed of a planet depends on its distance from the sun new version available! (3.0.119) get it now submit 18.07: module exam description
Answers: 3

Chemistry, 22.06.2019 23:00
Arectangle has a diagonal 20 inches long that forms angles of 60 and 30 with the sides. find the perimeter of the rectangle. for geometry
Answers: 3

Chemistry, 23.06.2019 01:00
Imagine if during the cathode ray experiment, the size of the particles of the ray was the same as the size of the atom forming the cathode. which other model or scientific observation would have also been supported? 1. this would support dalton's postulates that proposed the atoms are indivisible because no small particles are involved. 2. this would support bohr's prediction about electrons moving in orbits having specific energy. 3. this would support bohr's prediction about electrons being randomly scattered around the nucleus in the atom. 4. this would support dalton's postulates that proposed that atoms combine in fixed whole number ratios to form compounds.
Answers: 1
You know the right answer?
Magnesium (used in the manufacture of light alloys) reacts with iron(iii) chloride to form magnesium...
Questions

Physics, 08.10.2019 21:30

Mathematics, 08.10.2019 21:30


Mathematics, 08.10.2019 21:30

Biology, 08.10.2019 21:30

Mathematics, 08.10.2019 21:30

History, 08.10.2019 21:30

Chemistry, 08.10.2019 21:30





English, 08.10.2019 21:30


Mathematics, 08.10.2019 21:30

Computers and Technology, 08.10.2019 21:30

Mathematics, 08.10.2019 21:30

Biology, 08.10.2019 21:30

History, 08.10.2019 21:30

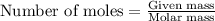 .....(1)
.....(1)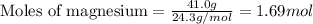
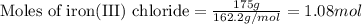
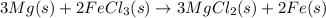
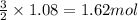 of magnesium
of magnesium