
Chemistry, 06.10.2019 04:00 zwbaby3693
At 298 k, the equilibrium concentration of o2 is 1.6 x 10-2 m, and the equilibrium concentration of o3 is 2.86 x 10-28 m. what is the equilibrium constant of the reaction at this temperature?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:00
During which movies do spring tides new moon first quarter waxing gibbous waxing
Answers: 1

Chemistry, 22.06.2019 02:10
What approach is required to balance the objectives of sustainable development? balancing the objectives of sustainable development requires a(n) .
Answers: 3

Chemistry, 23.06.2019 04:00
Calculate the mass of 0.750 mol of the following substance. na3po4. , i'm not quite sure on how to set up the problem to solve! : (
Answers: 1

Chemistry, 23.06.2019 06:10
How much would the freezing point of water decrease if 4 mol of nacl were added to 1 kg of water (kf=1.86 degrees c/(mol/kg) for water and i=2 for nacl a- 7.44 degrees c b- 14.88 c 3.72 d 1.86
Answers: 1
You know the right answer?
At 298 k, the equilibrium concentration of o2 is 1.6 x 10-2 m, and the equilibrium concentration of...
Questions

Computers and Technology, 28.02.2021 09:30

Social Studies, 28.02.2021 09:30


Mathematics, 28.02.2021 09:30




History, 28.02.2021 09:30

Mathematics, 28.02.2021 09:30

World Languages, 28.02.2021 09:30


Mathematics, 28.02.2021 09:30

Mathematics, 28.02.2021 09:30

Chemistry, 28.02.2021 09:30



Mathematics, 28.02.2021 09:30

Mathematics, 28.02.2021 09:30

Mathematics, 28.02.2021 09:30

Mathematics, 28.02.2021 09:30


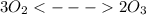
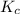 is the equilibrium constant and is defined as products concentration over reactant concentration and the coefficient is raised to its power. Thus we have the
is the equilibrium constant and is defined as products concentration over reactant concentration and the coefficient is raised to its power. Thus we have the ![K_c = \frac {[Products concentration]}{[Reactants concentration]}](/tpl/images/0291/9821/c00b5.png)
![K_c =\frac {[O_3 ]^2}{[O_2 ]^3}](/tpl/images/0291/9821/20678.png)
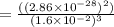
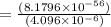
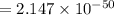 (Answer)
(Answer)

