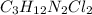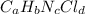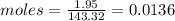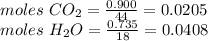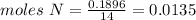Chemistry, 04.10.2019 20:20 estrellagutierrez12
Compound x contains only carbon, hydrogen, nitrogen, and chlorine. when 1.00 g of x is dissolved in water and allowed to react with excess silver nitrate, agno3, all the chlorine in x reacts and 1.95 g of solid agcl is formed. when 1.00 g of x undergoes complete combustion, 0.900 g of co2 and 0.735 g of h2o are formed. what is the empirical formula of x?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
This drawing shows a human body system. what is the primary function of this body system?
Answers: 3


Chemistry, 22.06.2019 18:30
The table lists the lattice energies of some compounds.compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf.the lattice energy increases as the cations get larger, as shown by lif and licl.the lattice energy decreases as cations get smaller, as shown by nacl and naf.the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3

You know the right answer?
Compound x contains only carbon, hydrogen, nitrogen, and chlorine. when 1.00 g of x is dissolved in...
Questions

English, 17.08.2021 20:40

Mathematics, 17.08.2021 20:40



English, 17.08.2021 20:40

English, 17.08.2021 20:40

Health, 17.08.2021 20:40




History, 17.08.2021 20:40

Mathematics, 17.08.2021 20:40




Geography, 17.08.2021 20:40


English, 17.08.2021 20:40