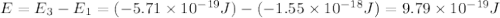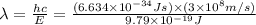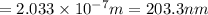Chemistry, 06.10.2019 05:30 kaylarae1930
Consider the following energy levels of a hypothetical atom: e4 −1.21 × 10−19 j e3 −5.71 × 10−19 j e2 −1.05 × 10−18 j e1 −1.55 × 10−18 j (a) what is the wavelength of the photon needed to excite an electron from e1 to e4? (b) what is the energy (in joules) a photon must have in order to excite an electron from e2 to e3? (c) when an electron drops from the e3 level to the e1 level, the atom is said to undergo emission. calculate the wavelength of the photon emitted in this process.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 17:00
The arrangement of particles is most ordered in a sample of
Answers: 1


Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3
You know the right answer?
Consider the following energy levels of a hypothetical atom: e4 −1.21 × 10−19 j e3 −5.71 × 10−19 j e...
Questions

Mathematics, 15.11.2021 01:30

English, 15.11.2021 01:30

Chemistry, 15.11.2021 01:30


Social Studies, 15.11.2021 01:30


Mathematics, 15.11.2021 01:30


Computers and Technology, 15.11.2021 01:30


Mathematics, 15.11.2021 01:30

Physics, 15.11.2021 01:30




English, 15.11.2021 01:30

Mathematics, 15.11.2021 01:30

Mathematics, 15.11.2021 01:30

Mathematics, 15.11.2021 01:30

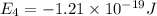
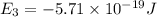
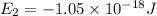
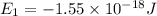
 to
to 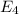 .
.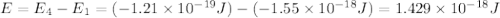
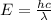
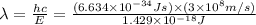
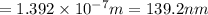
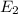 to
to 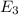 .
.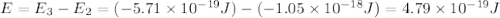
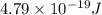 is the energy a photon to excite an electron from
is the energy a photon to excite an electron from