
Decide which of the following statements are true and which are false about equilibrium systems: a large value of k means the equilibrium position lies far to the right. for the following reaction: h2(g) + f2(g) ⇌ 2hf(g) the values of k and kp are not the same. the value of k at constant temperature does not depend on the amounts of reactants and products that are mixed together initially. for the following reaction: caco3(s) ⇌ cao(s) + co2(g) the [caco3] appears in the denominator of the equilibrium expression. for a reaction with k > > 1, the rate of the forward reaction is less than the rate of the reverse reaction at equilibrium.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 13:30
Which of the following natural processes is most likely to support the formation of an underwater sinkhole? a pollution buildup from deposited minerals b limestone cave collapsing due to changes in sea level c erosion of large amounts of sand moved by ocean waves d oxidation of rock formed by chemical weathering
Answers: 1

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1
You know the right answer?
Decide which of the following statements are true and which are false about equilibrium systems: a l...
Questions


Mathematics, 05.11.2019 22:31


Mathematics, 05.11.2019 22:31




English, 05.11.2019 22:31

History, 05.11.2019 22:31




History, 05.11.2019 22:31



Mathematics, 05.11.2019 22:31


Biology, 05.11.2019 22:31


Mathematics, 05.11.2019 22:31

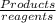
![K= \frac{[C]^{c}[D]^{d}}{[A]^{a}[B]^{b}}](/tpl/images/0292/7295/c008c.png)
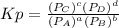
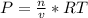 we know that
we know that 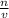 is the molar concentration. When we replace P in the expression for Kp we get:
is the molar concentration. When we replace P in the expression for Kp we get:
![Kp= \frac{[C]^{c}*(RT)^{c}[D]^{d}*(RT)^{d}}{[A]^{a}*(RT)^{a}[B]^{b}*(RT)^{b}}](/tpl/images/0292/7295/8e3a8.png)
![Kp= \frac{[C]^{c}[D]^{d}}{[A]^{a}[B]^{b}}*\frac{(RT)^{c+d}}{(RT)^{a+b}}](/tpl/images/0292/7295/243b8.png)
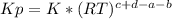
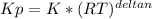
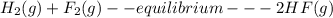
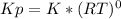
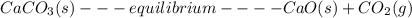
![K= [CO_{2}]](/tpl/images/0292/7295/89ef2.png)
![[CaCO_{3}]](/tpl/images/0292/7295/84a01.png) is not include in the expression.
is not include in the expression. 

