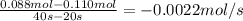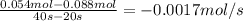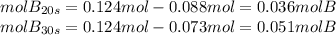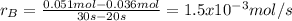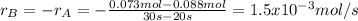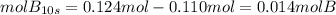Aflask is charged with 0.124 mol of a and allowed to react to from b according to the reaction: a(g) -> b(g). the following drata are obtained for [a] as the reaction proceeds:
time: 0.00 10.0 20.0 30.0 40.0
moles of a: 0.124 0.110 0.088 0.073 0.054
a) what is the average rate of disapperance of a between 10s and 20s?
b) what is the avergae rate of disappearance of a between 20s and 40s?
c) what is the average rate of appearance of b between 20s and 30s?
d) how many moles of b are present at 10s?
e) how many moles of b are present at 30s?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
What are the major products produced in the combustion of c10h22 under the following conditions? write balanced chemical equations for each. a. an excess of oxygen b. a slightly limited oxygen supply c. a very limited supply of oxygen d. the compound is burned in air
Answers: 2

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1


Chemistry, 22.06.2019 22:30
Calculate the concentration of all species in a 0.165 m solution of h2co3.
Answers: 1
You know the right answer?
Aflask is charged with 0.124 mol of a and allowed to react to from b according to the reaction: a(g...
Questions

Mathematics, 01.12.2021 03:20

Computers and Technology, 01.12.2021 03:20

Chemistry, 01.12.2021 03:20

Chemistry, 01.12.2021 03:20



Mathematics, 01.12.2021 03:20

Mathematics, 01.12.2021 03:20

Mathematics, 01.12.2021 03:20


Mathematics, 01.12.2021 03:20




Physics, 01.12.2021 03:20

Computers and Technology, 01.12.2021 03:20


Social Studies, 01.12.2021 03:20

Mathematics, 01.12.2021 03:20