Chemistry, 06.10.2019 09:02 eastonstelter
One step in the isolation of pure rhodium metal (rh) is the precipitation of rhodium(iii) hydroxide from a solution containing rhodium(iii) sulfate according to the following balanced chemical equation: rh₂(so₄)₃(aq) + 6naoh(aq) → 2rh(oh)₃(s) + 3na₂so₄(aq) of 0.730 g of rhodium(iii) hydroxide is produced, what mass of sodium sulfate is also produced?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 09:00
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3

Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2
You know the right answer?
One step in the isolation of pure rhodium metal (rh) is the precipitation of rhodium(iii) hydroxide...
Questions


English, 22.07.2020 21:01

Mathematics, 22.07.2020 21:01



Mathematics, 22.07.2020 21:01

History, 22.07.2020 21:01

Physics, 22.07.2020 21:01

Spanish, 22.07.2020 21:01

Physics, 22.07.2020 21:01






Health, 22.07.2020 21:01




Arts, 22.07.2020 21:01

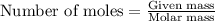 .....(1)
.....(1)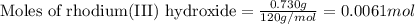
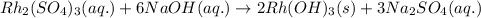
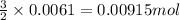 of sodium sulfate is also produced
of sodium sulfate is also produced