
Chemistry, 06.10.2019 09:02 oliviaschmitt0
The blood alcohol (c2h5oh) level can be determined by titrating a sample of blood plasma with an acidic potassium dichromate solution, resulting in the production of cr3+(aq) and carbon dioxide. the reaction can be monitored because the dichromate ion (cr2o72−) is orange in solution, and the cr3+ ion is green. the unbalanced redox equation is the following. cr2o72−(aq) + c2h5oh(aq) → cr3+(aq) + co2(g) if 31.91 ml of 0.0613 m potassium dichromate solution is required to titrate 33.8 g of blood plasma, determine the mass percent of alcohol in the blood.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no2]2 ? second order 3/2 order third order zero order none of the listed answers are correct
Answers: 3


Chemistry, 22.06.2019 07:00
Which set of characteristics best describes igneous rock? a) largest type of rock, made of organic matter, hardest type of rock b) least abundant type of rock, made of other rocks, made mostly of minerals c) found on all continents, contains wavy bands of stripes, contains fossils d) most abundant type in earth's crust, made of magma/lava, contains no fossils
Answers: 1

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1
You know the right answer?
The blood alcohol (c2h5oh) level can be determined by titrating a sample of blood plasma with an aci...
Questions

Mathematics, 06.07.2019 21:00

Mathematics, 06.07.2019 21:00


History, 06.07.2019 21:00

Mathematics, 06.07.2019 21:00

History, 06.07.2019 21:00






Social Studies, 06.07.2019 21:00

Mathematics, 06.07.2019 21:00



History, 06.07.2019 21:00




Mathematics, 06.07.2019 21:00

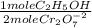 = 9,780x10⁻⁴ moles of C₂H₅OH
= 9,780x10⁻⁴ moles of C₂H₅OH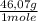 = 0,04506 g of C₂H₅OH
= 0,04506 g of C₂H₅OH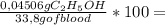 0,1333% of alcohol in the blood
0,1333% of alcohol in the blood

