
Chemistry, 05.10.2019 02:10 Whitehouse9
Write the chemical equation and the ka expression for the acid dissociation of each of the following acids in aqueous solution. first show the reaction with h+(aq) as a product and then with the hydronium ion. part a c6h5cooh. show the reaction with h+(aq) as a product. express your answer as a chemical equation. identify all of the phases in your answer. nothing request answer part b write the ka expression for the acid dissociation from the previous part. write the expression for the acid dissociation from the previous part. 1[h+][c6h5coo−] [c6h5cooh][h+][c6h5coo−] [h+][c6h5coo−][c6h5cooh] [h+][c6h5coo−]

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:30
The isotonic saline solution described in part a is connected to an unknown solution via a semipermeable membrane, the unknown solution level drops. based on this information, what can be said about these two solutions?
Answers: 1

Chemistry, 22.06.2019 01:40
Which characteristic of water it form droplets? a. low specific heat b. nonpolar structure c. high surface tension d. ability to dissolve substances
Answers: 1

Chemistry, 22.06.2019 09:00
How are isotopes of the same chemical element alike? how are they different?
Answers: 1

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3
You know the right answer?
Write the chemical equation and the ka expression for the acid dissociation of each of the following...
Questions


Mathematics, 07.06.2020 23:57




Social Studies, 07.06.2020 23:57


Mathematics, 07.06.2020 23:57



Mathematics, 07.06.2020 23:57




Mathematics, 07.06.2020 23:57

English, 07.06.2020 23:57

Mathematics, 07.06.2020 23:57


Mathematics, 07.06.2020 23:57

Computers and Technology, 07.06.2020 23:57

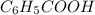
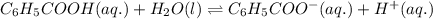
![K_a=\frac{[C_6H_5COO^-][H^+]}{[C_6H_5COOH]}](/tpl/images/0287/8245/438a5.png)


