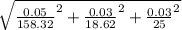Atitration is performed to calculate the concentration of a solution of a monoprotic acid. the buret is filled with a standardized solution of 158.32 ± 0.05 mm naoh. the initial volume is recorded as 0.14 ml. 25.00 ml of the unknown acid solution are pipetted into an erlenmeyer flask and the solution is titrated to a phenolphthalein endpoint. the final buret reading is 18.76 ml. assuming that the error in each volumetric measurement (buret and pipet) is ±0.03 ml, calculate the concentration of the acid (mm) and use propagation of error to estimate its uncertainty.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 04:00
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 22.06.2019 09:00
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1

Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2
You know the right answer?
Atitration is performed to calculate the concentration of a solution of a monoprotic acid. the buret...
Questions

History, 07.12.2019 07:31


Biology, 07.12.2019 07:31

Mathematics, 07.12.2019 07:31

Mathematics, 07.12.2019 07:31



History, 07.12.2019 07:31

Mathematics, 07.12.2019 07:31






Biology, 07.12.2019 07:31