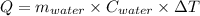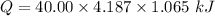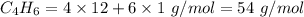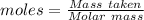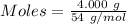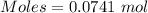Chemistry, 06.10.2019 10:01 Jazzy4real
4.000 g of compound x with molecular formula c4h6 are burned in a constant-pressure calorimeter containing 40.00 kg of water at 25 °c. the temperature of the water is observed to rise by 1.065 °c. (you may assume all the heat released by the reaction is absorbed by the water, and none by the calorimeter itself.) calculate the standard heat of formation of compound x at 25 °c. be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3

Chemistry, 22.06.2019 20:40
What effect would average population growth have on land usage? a. urban use of land would rise to more than 30 percent of available land. b. industrial use of land would rise to more than 30 percent of available land. c. the percentage of available land used as cropland would stay the same. d. cropland would fall to about 10 percent of available land.
Answers: 1

Chemistry, 22.06.2019 22:00
11) burning your hand when accidentally touching a hot plate is an example of which heat transfer? a. conduction b. convection c. radiation d. none of these
Answers: 2
You know the right answer?
4.000 g of compound x with molecular formula c4h6 are burned in a constant-pressure calorimeter cont...
Questions

Computers and Technology, 19.12.2020 08:30


Mathematics, 19.12.2020 08:30


History, 19.12.2020 08:30


Mathematics, 19.12.2020 08:30



Mathematics, 19.12.2020 08:30

Mathematics, 19.12.2020 08:30

Biology, 19.12.2020 08:30

Mathematics, 19.12.2020 08:30

Physics, 19.12.2020 08:30


Health, 19.12.2020 08:30



Chemistry, 19.12.2020 08:30

Mathematics, 19.12.2020 08:30

 = 1.065 °C
= 1.065 °C