
Chemistry, 06.10.2019 10:00 cjrogers4401
You are asked to prepare 500 ml 0.300 m500 ml 0.300 m acetate buffer at ph 4.904.90 using only pure acetic acid ( mw=60.05 g/mol, mw=60.05 g/mol, pka=4.76), pka=4.76), 3.00 m naoh,3.00 m naoh, and water. answer the questions regarding the preparation of the buffer. 1. how many grams of acetic acid will be needed to prepare the 500 ml buffer? note that the given concentration of acetate refers to the concentration of all acetate species in solution.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2

Chemistry, 22.06.2019 10:50
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments,solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1

Chemistry, 22.06.2019 19:00
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2

Chemistry, 22.06.2019 19:50
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1
You know the right answer?
You are asked to prepare 500 ml 0.300 m500 ml 0.300 m acetate buffer at ph 4.904.90 using only pure...
Questions

Mathematics, 26.07.2019 00:30

English, 26.07.2019 00:30





Spanish, 26.07.2019 00:30

History, 26.07.2019 00:30

English, 26.07.2019 00:30


Biology, 26.07.2019 00:30

Mathematics, 26.07.2019 00:30

History, 26.07.2019 00:30



History, 26.07.2019 00:30


Physics, 26.07.2019 00:30


![\frac{[A^{-}]}{[HA]}](/tpl/images/0293/1269/5980f.png)
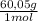 = 9,0 g of acetic acid
= 9,0 g of acetic acid

