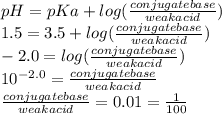Chemistry, 05.10.2019 04:10 meababy2009ow9ewa
Use the henderson-hasselbalch equation and your knowledge of ionization to you answer this question. aspirin is a weak acid with a pka of 3.5 that is absorbed more effectively in the stomach than the small intestine. the ph of your stomach is around 1.5 and the ph of your small intestine is approximately 6.0. is aspirin absorbed more readily when it is protonated or deprotonated? what is the approximate ratio of conjugate base to acid when it is absorbed more readily?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Which of the following happens during cell division? (a) energy is created (b) waste is eliminated (c) carbon dioxide is released (d) damaged cells are replaced
Answers: 1


Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1

Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1
You know the right answer?
Use the henderson-hasselbalch equation and your knowledge of ionization to you answer this question...
Questions

English, 03.08.2019 19:00

Mathematics, 03.08.2019 19:00



Mathematics, 03.08.2019 19:00








Mathematics, 03.08.2019 19:00




History, 03.08.2019 19:00